Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Suzuki, Hideya*; Naganawa, Hirochika; Tachimori, Shoichi
Solvent Extraction and Ion Exchange, 21(4), p.527 - 546, 2003/03
Times Cited Count:12 Percentile:45.03(Chemistry, Multidisciplinary)no abstracts in English
 medium in a HNO
medium in a HNO /supercritical CO
/supercritical CO -tributyl phosphate system
-tributyl phosphate systemMeguro, Yoshihiro; ; Yoshida, Zenko
Analytical Chemistry, 70(7), p.1262 - 1267, 1998/00
Times Cited Count:52 Percentile:85.99(Chemistry, Analytical)no abstracts in English
Shibata, Atsuhiro; P.Y.COR*; *; ; ; *; Tanaka, Yasumasa
PNC TN8410 95-112, 100 Pages, 1995/05
PNC has developed the TRUEX process to recover transuranium elements from high level liquid waste. This process uses CMPO as extracting solvent. DIAMEX process which uses Diamide has been developed bY CEA in France. The aim of basic studies was to get some comparison data between CMPO and Diamide. We have conducted the experiments regarding properties of Ln(III) extraction and behavior of third phase formation. Also, we have investigated properties of Ln(III) extraction with TBP and DBBP to compare bidentate ligand with monodentate ligand. This study was conducted as a frame of PNC/CEA collaboration on minor Actinide partitioning. The conclusions drawn from our studies can be summarized as follows; (1)Trivalent Lanthanides extraction reaction. CMPO, TBP and DBBP ; Ln + 3NO
+ 3NO + 3Extractant
+ 3Extractant 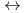 Ln(NO
Ln(NO )
)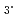 3Extractant. Diamide; Ln
3Extractant. Diamide; Ln + 3NO
+ 3NO + m Diamide
+ m Diamide 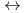 Ln(NO
Ln(NO )
)
 m Diamide : m=O.7
m Diamide : m=O.7 1.8. (2)Extraction equilibrium constant. Apparent extraction equilibrium constants were calculated from the trivalent Lanthanides extraction reaction. The value of the constants were CMPO
1.8. (2)Extraction equilibrium constant. Apparent extraction equilibrium constants were calculated from the trivalent Lanthanides extraction reaction. The value of the constants were CMPO  Diamide
Diamide 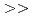 DBBP
DBBP  TBP.CMPO has high extraction efficiency for trivalent metals such as Am, but the back extraction is difficult in nitric acid media. Diamide indicated better behavior about both extraction and back extraction. (3)Separation Factors. We got separation factors (SFs) for intra-group in nitric acid. So SFs for CMPO and Diamide were nearly one, that these solvents could not separate each Lanthanides elements. The other hand, SFs for TBP and DBBP were 2
TBP.CMPO has high extraction efficiency for trivalent metals such as Am, but the back extraction is difficult in nitric acid media. Diamide indicated better behavior about both extraction and back extraction. (3)Separation Factors. We got separation factors (SFs) for intra-group in nitric acid. So SFs for CMPO and Diamide were nearly one, that these solvents could not separate each Lanthanides elements. The other hand, SFs for TBP and DBBP were 2  10. (4)Behavior of third phase formation. When using CMPO or Diamide, even in the case of extraction of nitric acid, third phase formation was found. When third phase was formed, the nitric acid concentration in aqueous phase was 6 N for CMPO, 4 N for Diamide. If Diamide diluted with Decaline was used, third phase wasn't found ...
10. (4)Behavior of third phase formation. When using CMPO or Diamide, even in the case of extraction of nitric acid, third phase formation was found. When third phase was formed, the nitric acid concentration in aqueous phase was 6 N for CMPO, 4 N for Diamide. If Diamide diluted with Decaline was used, third phase wasn't found ...
;
PNC TN8420 95-002, 140 Pages, 1995/01
In the field of uranium ore refining and spent fuel reprocessing, a lot of extractants capable of recovering actinides have been innovated, and some of which have been developed in actual industrial scale processes. Extractability against trivalent actinides on each extractants were evaluated in this report. And as for the compounds which showed excellent extractability for Americium, their extraction characteristics for trivalent actinides, such as extraction behavior, mechanism, ligand structure and conformation of complexes, were evaluated, and filed as data-base. Based on these works, some of extractant were newly identified as candidate to apply Americium recovering. The most appropriate compounds for trivalent actinide separation can be generally found in the neutral bidentate organic compound groups: Especially, some derivatives belongs to Diphosphineoxide, Carbamoyl-methylphosphine oxide and Propandiamide were superior to give sufficiently excellent property even in higher nitric acid(1 3M) solutions. Innovative research towards developping new extractants and extraction system seems to be directed to ; (1)investigate new types of extractant sustained by a different kind of complexing mechanism, (2)evaluate synergistic effect of adducts, diluents even including conventional extractants.
3M) solutions. Innovative research towards developping new extractants and extraction system seems to be directed to ; (1)investigate new types of extractant sustained by a different kind of complexing mechanism, (2)evaluate synergistic effect of adducts, diluents even including conventional extractants.
 system, I; Extraction behaviour of Th(IV) and U(VI) with the TBP/XAD-4 resin
system, I; Extraction behaviour of Th(IV) and U(VI) with the TBP/XAD-4 resinKimura, Takaumi
Journal of Radioanalytical and Nuclear Chemistry, 141(2), p.295 - 306, 1990/00
Times Cited Count:18 Percentile:83.14(Chemistry, Analytical)no abstracts in English